Abstract
BACKGROUND:
CD74 is a transmembrane glycoprotein involved in MHC II protein formation and transport. STRO-001 is a novel antibody drug conjugate (ADC) comprised of a human aglycosylated anti-CD74 IgG1 antibody (SP7219) genetically incorporating the non-natural amino acid para-azidomethy-L-phenyalanine (pAMF) to enable the site-specific conjugation of a non-cleavable maytansinoid linker-warhead. Highly efficient and precise site-specific conjugation enabled by Sutro's cell-free antibody synthesis technology produced a well-defined homogeneous ADC with a drug-antibody ratio (DAR) of 2. Conjugation sites were selected based on the highest in vitro and in vivo stability.
METHODS:
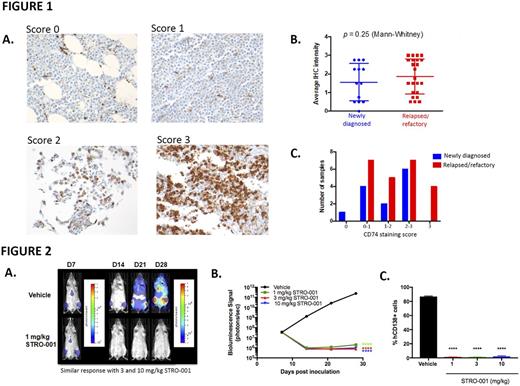
Biotinylated SP7219 was used for immunohistochemistry (IHC). Bone marrow (BM) biopsy samples from patients with multiple myeloma (MM) from the UCSF Dept. of Laboratory Medicine were used after appropriate permissions were obtained. CD74 expression in plasma cells was measured as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; 3+, strong staining (Figure 1A). Plasma cells were identified based on morphology and paired CD138+ staining. Two pathologists blinded to the patient's clinical history and pathology results independently reviewed IHC specimens. SP7219 conjugated to a fluorescent dye (DBCO-Alexa647) was used for detection and quantitation of CD74 expression on MM cell lines. STRO-001 was used to determine the EC50 and the proportion of MM cells killed (span killing) in an in vitro viability assay. The anti-tumor activity of STRO-001 was evaluated in the disseminated ARP-1 and MM.1S MM models. In vivo bioluminescence imaging (BLI) for animals bearing luciferase-expressing MM.1s (MM.1s-luc) cells was performed using an IVIS Spectrum. BLI images were collected 7, 14, 21, and 28 days post-tumor cell inoculation.
RESULTS:
Overall, CD74 expression levels were not significantly different between newly diagnosed MM (n=13) and relapsed or refractory MM (n=24) (Figure 1B). Of 37 total samples analyzed, only 1 sample lacked detectable CD74 and the majority (67%) of relapsed/refractory patients expressed CD74 levels higher than 1+ by IHC (Figure 1C). In vitro cytotoxicity assays show nanomolar potency of STRO-001 in five MM cell lines: MC/CAR (EC50 0.8 nM), ARD (EC50 ~7.0 nM), MM.1S (EC50 10-11 nM), U266B1 (EC50 8.5-9.3 nM), and ARP-1 (EC50 4.3-22 nM). CD74 cell surface expression is required for STRO-001 cytotoxic activity but expression level, measured by antibody-binding capacity, does not correlate strongly with in vitro potency (R2=0.5837 for MM cell lines). STRO-001 inhibits the growth of CD138+ plasma cells in BM and formation of visceral tumors (p=0.002 for kidney; p<0.0001 for ovary) after 4 weekly doses of 3 mg/kg in the ARP-1 disseminated MM xenograft model in severe combined immune deficient (SCID) mice. STRO-001 dosed at 3 mg/kg and 10 mg/kg weekly x 3 also eradicates malignant BM plasma cells by day 32 post-inoculation (p<0.0001) and prolongs survival in the MM.1S disseminated model in NOD SCID gamma (NSG) mice. At termination of the study, 129 days post-inoculation, 100% of the STRO-001 treated animals survived and showed no evidence of disease with no CD138+ cells in their BM, while mean survival of control animals was 35 days with almost 50% of their BM containing myeloma cells. Additionally, BLI enabled noninvasive quantitation of tumor burden in MM.1S-luc-bearing NSG mice. Single doses of 1, 3, and 10 mg/kg STRO-001 (administered on day 7 post-inoculation) resulted in eradication of myeloma by day 28 based on BLI (Figure 2A and 2B) and quantification of CD138+ cells in BM (p<.0001) (Figure 2C).
CONCLUSION:
CD74 expression was detected in most MM samples examined in this study. Expression is heterogeneous and observed in treatment-naïve and heavily pre-treated patients. STRO-001 demonstrates potent in vitro cytotoxicity in MM cell lines and reduces tumor burden in MM xenograft models, including prolongation of survival in the MM.1S model. Based on these encouraging observations, STRO-001 is advancing to the clinic for the treatment of myeloma and other B-cell malignancies.
Embry: Sutro Biopharma: Employment. Li: Sutro Biopharma: Employment. Yu: Sutro Biopharma: Employment. Abrahams: Sutro Biopharma: Employment. DeAlmeida: Sutro Biopharma: Employment. Krimm: Sutro Biopharma: Employment. Matheny: Sutro Biopharma: Employment. Kline: Sutro Biopharma: Employment. Yam: Sutro Biopharma: Employment. Stafford: Sutro Biopharma: Employment. Wiita: Sutro Biopharma: Research Funding; TeneoBio, Inc.: Research Funding. Hallam: Sutro Biopharma: Employment. Lupher: Sutro Biopharma: Employment. Molina: Sutro Biopharma: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal